We recently attended two battery conferences in Carlsbad, CA (NAATBatt) and Orlando, FL (International Battery Seminar) and were struck by both the amount and array of new materials-based approaches companies are taking towards improving legacy, lithium-ion batteries (LIB) and alternative battery technologies. Our CCO Mas Fukumoto recently shared some impressions here and additional takeaways here from these conferences. We were glad to be able to spend some time discussing the challenges that battery and battery materials manufacturers face, and how Peaxy Lifecycle Intelligence (PLI) can help streamline research and development processes. I would like to share some of those insights here.
Problem Statement
Let’s begin by using a concrete example of how battery analytics can aid cell materials development, which is a topical issue for many folks in the field. Clearly, the kinetics of Li⁺ diffusion and transmission strongly affect both the performance and safety properties of a LIB. To attempt to improve both, perhaps we believe that by increasing the uniformity of the cathode material, we can allow for faster charging and discharging. Alternatively, we may believe that we can take advantage of an silicon oxide (SiO) anode – which is known to have high specific capacity but poor intrinsic conductivity – by introducing highly conductive layers of silicon carbide/carbide (SiC/C) for better performance. Or perhaps we have pursued one, or several, of a host of the many, many other materials based approaches seeing active development, including solid state cathodes, various cathode fabrication techniques, solid state electrolytes, flexible separators, all Si anodes, SiC anodes of many different flavors, etc., though not all of these earmark the Li⁺ diffusion as the primary figure of merit.
Regardless of the approach, the problem statement is: how sensitive is our Li⁺ diffusion constant (often in the 10⁻¹¹ to 10⁻¹³ cm²·s⁻¹ range at ambient temperatures) to the materials used in the cell.
Researchers can approach this problem by: (1) making the material (thereby creating data, like material components; reaction time; milling time; sintering temperature, time, occasionally also atmosphere; etc.); (2) characterizing the material (thereby creating data from measurements like mass; appearance; etc and metrology like SEM, TGA, PSD, BET, FTIR, OES, NMR, etc.); (3) fabricating the cell (thereby creating data like slurry composition; cathode, anode, electrolyte, separator; press force; operator; etc.) and (4) analyzing the cell (thereby creating data like electrochemical test data of many flavors). A data analysis follows these four broadly defined steps, and that is the challenge we address with our customers:
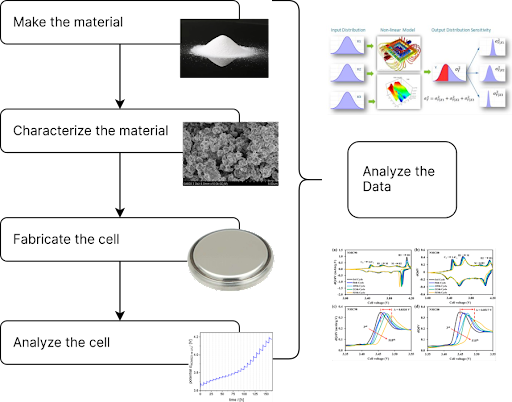
To address the problem statement, let’s break it down starting at the end. First, we certainly need the Li⁺ diffusion constant, and there are multiple ways to capture this including cyclic voltammetry (CV) and my favorite, galvanostatic intermittent titration technique (GITT). Other folks might use an analysis of Electrochemical Impedance Spectroscopy (EIS) or even Electron Parametric Resonance (EPR), (assuming access to the equipment!). Regardless, the technique results in a file which must be analyzed, so we have the first challenge (see table below).
Next, we need to relate that measurement to the actual material being used, what we call the “data threading problem.” Organizations spend an agonizingly large amount of time doing exactly this, typically by creating a spreadsheet to help them track the material as it makes its way through slurries into sheets and into cells which then are plugged into one of the hundreds of cycler channels which many typically companies use. This often involves spending a large amount of time aggregating data from multiple sources into a single view.
Finally, for the third challenge, we need to go back and identify the properties of our material from the metrology files, often a highly specialized process involving things like applying density functional theory to Brunauer-Emmet-Teller measurements. In general we could call this step refinement, as in the refinement of an X-Ray Diffraction plot to determine crystal structure parameters, for example.
The Solution
PLI is a software solution uniquely suited to address these three challenges given it was designed specifically for battery R&D and monitoring, and implemented by a team whose experience spans over a dozen different battery chemistries. PLI addresses the challenges as follows:
Architecturally, this is possible given the software design, which allows for sophisticated configuration, all within a framework that is generally applicable. One way to explain the concept would be to see the diagram below:
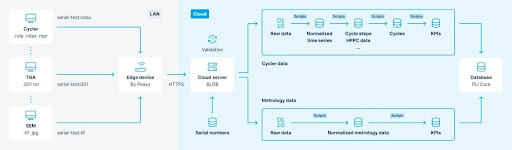
Metrology files from materials and cell testing are ingested into the system. The two major ‘tricks’ are to enforce a hierarchical serial number scheme on the assets. This allows for threading which is arguably, from an organizational perspective, the larger challenge in addressing the problem statement.
Having scripts which trigger at every step increases efficiency for file-based metrology. Take image analysis as our first example here. Many folks, particularly of a certain age, are familiar with using ImageJ or Fiji to construct a somewhat automated analysis (though Python-based approaches continue to gain momentum on these Java packages). With PLI as architected, the image analysis is a script that runs in the cloud and is optimized for the use case. Particle size distributions, defect detection and feature size calculations are examples of the types of analysis that PLI can automate. The same logic applies to the calculation of the Li⁺ diffusion constant. Although almost all battery cyclers are capable of running a GITT test, most don’t provide the analysis, but PLI has a script for that!
Of course, this approach is not limited to relating diffusion constants to material parameters. PLI is used throughout the battery life cycle, as has been described by past issues. The approach is not limited to LIB, as we are active with a variety of legacy and novel chemistries.
Conclusion
As we’ve seen in the examples above, battery analytics can significantly enhance the development of cells and cell materials by providing a data-driven approach to research and development (R&D). For battery and battery materials companies, the integration of advanced analytics on top of existing systems can lead to a deeper understanding of cell behavior under various conditions, enabling the identification of optimal material compositions and structures. This ultimately can result in improved battery performance, longevity, and safety.
As is evidenced by our own customers using PLI, a battery analytics solution can also streamline the R&D process by predicting outcomes of material changes, thus reducing the need for extensive trial-and-error experimentation. This not only accelerates the pace of innovation but also reduces costs associated with testing. In an industry driven by increasing competition and cost pressures, battery analytics serves as a powerful tool to drive the creation of more efficient and cost-effective battery solutions, and provide a needed edge in the marketplace.

